-
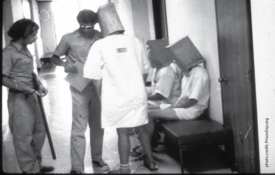
How the Classics Changed Research Ethics
Some of history’s most controversial psychology studies helped drive extensive protections for human research participants. Some say those reforms went too far.
-
Using an Automated Wizard to Process Minimal-Risk Research
A user-friendly decision tool could help researchers identify minimal-risk research, easing the administrative burden on both investigators and institutional reviewers.
-

Revision to the Common Rule: Implications for Behavioral and Social Sciences Research
The various provisions of the US government’s revised rule on human subjects research will provide increased flexibility and less burden for behavioral and social sciences researchers. William T. Riley and Farheen Akbar from the Office of Behavior and Social Sciences Research explain.
-
NIH Simplifies IRB Procedures for Multisite Studies
Multisite research collaborations can lead to significant discoveries, but they are also a challenge for many reasons, including logistical ones. The National Institutes of Health (NIH) have introduced a new policy to streamline one aspect
-

Finding a Home for Your Science
Learn the best places and practices for storing, managing, and sharing your data in this guide to online data repositories.
-
HHS to Hold Town Hall Meeting on Proposed ‘Common Rule’ Revisions
The US government’s Office for Human Research Protections (OHRP) will hold a public Town Hall Meeting on October 20, 2015 in Washington, DC to answer questions about proposed updates to the so-called Common Rule governing

